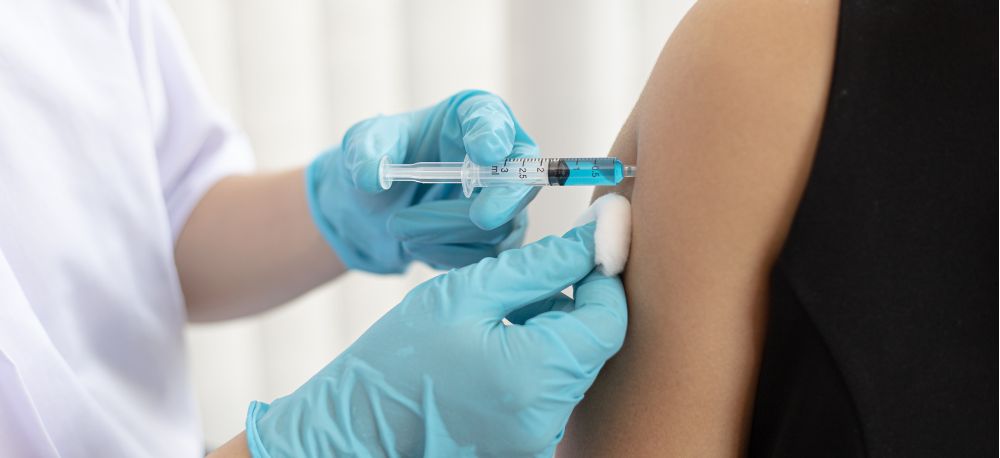
The European Committee for Treatment and Research in Multiple Sclerosis, in collaboration with the European Academy of Neurology, is pleased to announce the publication of a groundbreaking consensus statement, “European Committee for Treatment and Research in Multiple Sclerosis and European Academy of Neurology Consensus on Vaccination in People with Multiple Sclerosis: Improving Immunization Strategies in the Era of Highly Active Immunotherapeutic Drugs.”
Published on 9 June 2023 in the Multiple Sclerosis Journal and the European Journal of Neurology, this consensus statement marks the first first-ever European consensus on vaccination in pwMS, addressing the critical need for evidence-based guidelines on vaccination strategies for people with multiple sclerosis (pwMS) who are candidates for disease-modifying therapies (DMTs). By proposing the best vaccination strategy based on current evidence and expert knowledge, the aim is to standardise and harmonise immunisation practices in pwMS. This will significantly contribute to improving the overall well-being and quality of life for individuals living with multiple sclerosis.
“The publication of this consensus statement is a landmark achievement for the multiple sclerosis community. It represents a significant step forward in our collective efforts to enhance the management of people with multiple sclerosis. ECTRIMS is proud to present this evidence-based guidance, which will serve as a valuable resource for healthcare professionals, patients, and their families. We believe that by implementing these recommendations, we can optimise vaccination strategies, minimise risks, and ensure the best possible outcomes for individuals with MS.”
Mar Tintoré; ECTRIMS President
“This joint consensus statement is another milestone in the series of joint ECTRIMS and EAN activities. Recommendations on vaccination strategies for people with MS are primarily of utmost importance for individuals with MS as they contribute to ensuring the best management by their health care providers in this regard. In this sense, the core spirit of a joint ECTRIMS-EAN consensus is to afford “one voice” on behalf of all parties concerned. Moreover, the EAN contributes to boosting the dissemination of these consensus recommendations among and via its 45,000 member neurologists across Europe.”
Thomas Berger; EAN Scientific Committee Chair
Background and Purpose: With the advent of highly active immunotherapeutic drugs for pwMS, vaccination has become a crucial component of risk management strategies. Recognizing the importance of developing an evidence-based vaccination consensus, ECTRIMS and EAN embarked on an ambitious project to establish European guidelines for the vaccination strategy of pwMS who are candidates for DMTs.
Methods: A multidisciplinary working group, employing formal consensus methodology, led the comprehensive research efforts. Clinical questions were defined, taking into consideration the population, interventions, and outcomes related to all authorized DMTs and vaccines. A systematic literature search was conducted, and the quality of evidence was assessed using the Oxford Centre for Evidence-Based Medicine Levels of Evidence. Recommendations were formulated based on the quality of evidence and the risk-benefit balance.
Results: The working group addressed seven key questions, covering topics such as vaccine safety, vaccine effectiveness, global vaccination strategy, and vaccination in specific subpopulations (pediatric, pregnant women, elderly, and international travelers). A comprehensive narrative description of the evidence, including published studies, guidelines, and position statements, was provided. After three rounds of consensus, a total of 53 recommendations were agreed upon by the working group.
The consensus statement, “European Committee for Treatment and Research in Multiple Sclerosis and European Academy of Neurology Consensus on Vaccination in People with Multiple Sclerosis: Improving Immunization Strategies in the Era of Highly Active Immunotherapeutic Drugs,” is now available in the European Journal of Neurology and the Multiple Sclerosis Journal, also be accessed via the ECTRIMS and the EAN websites.
About ECTRIMS:
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) is the leading European organisation dedicated to the understanding and treatment of multiple sclerosis (MS). ECTRIMS promotes and fosters multidisciplinary research and education in MS and provides a platform for communication, collaboration, and dissemination of knowledge among healthcare professionals.
About EAN:
The European Academy of Neurology (EAN) is a non-profit, independent organization representing more than 45.000 individual members, as well as 47 European national neurological societies. EAN is dedicated to foster, support and promote clinical, research and educational excellence in neurology to improve patient care and outcome, as well as prevention of neurological disorders in Europe and beyond.
For media inquiries, please contact:
Naomi Smith; Communications and Marketing Manager | ECTRIMS
ectrims.communications@congrex.com